Transfluor® Assay on the iCyte™ Imaging Cytometer
- Kazuo Ozawa, Ph.D., Etsuo Shinohara, and Sachiko Karaki, Ph.D.
- Olympus Corporation, Genome Medical Business Division, Tokyo, Japan
- Atto corporation
Introduction
Transfluor® technology is a cell-based fluorescence assay system for screening G-protein coupled
receptors (GPCRs) developed by Norak Biosciences (Morrisville, NC, USA)1. The Transfluor assay
was developed based on the receptor desensitization mechanism. Upon ligand binding, GPCR is
activated by association with G protein and then desensitized by phosphorylation and arrestin binding followed by internalization and recycling. The desensitization mechanism is common to most GPCRs.

The Transfluor assay measures receptor activities by quantifying the redistribution of arrestin protein
from cytosol to GPCR on the cell
membrane and endosome. By labeling the
arrestin molecule with green fluorescence
protein (GFP), the Transfluor assay can
visually monitor the processes of
desensitization. Because the
desensitization is closely coupled to
activation of GPCR, changes in the
intracellular distribution of the arrestin
molecules reflect the active status of
GPCRs. By using a cell line transfected
with expression vectors of a particular
GPCR molecule and the arrestin-GFP, it is
possible to evaluate agonistic and
antagonistic activity of chemical
compounds towards specific GPCRs.
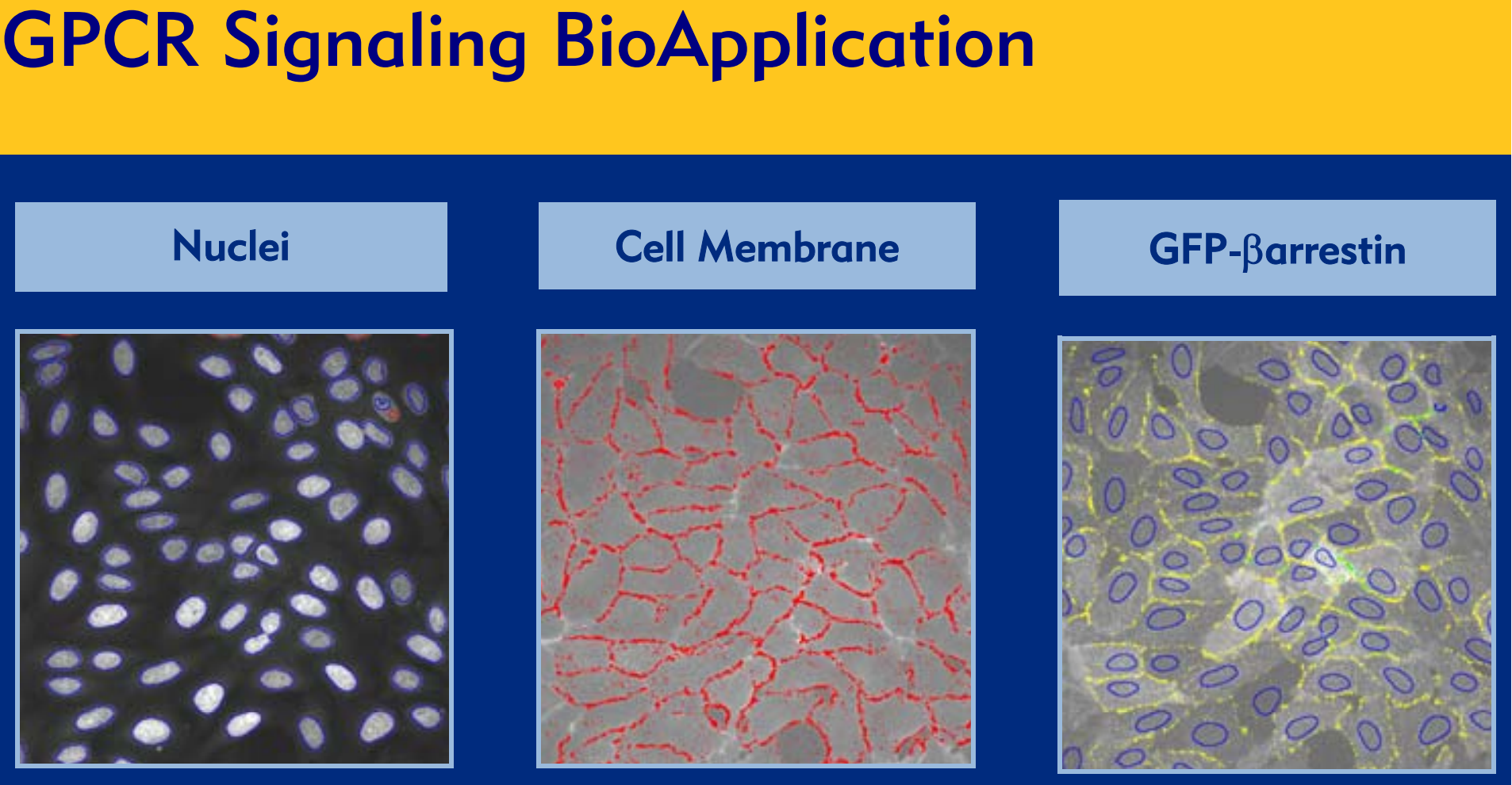
In the absence of agonist stimulation,
arrestin-GFP molecules are evenly
distributed throughout the cytosol
(Figure 1A).
Once GPCR is activated, intracellular
movement of the arrestin-GFP protein can
be observed under a fluorescence
microscope: toward clathrin-coated pit (Pittype signals, Figure 1B) within seconds; or
toward endocytic vesicles within minutes
(Vesicle-type signals, Figure 1C).
1A. Unstimulated
U-2 OS cells co-expressing arrestin-GFP plus wild-type
beta2-adrenergic receptor without receptor stimulation.
1B. Agonist-stimulated Pit-type signals
High-dose agonist-treated U2-OS cells co-expressing
arrestin-GFP and wild-type beta2-adrenergic receptor.
1C. Agonist-stimulated Vesicle-type signals
High-dose agonist-treated U2-OS cells co-expressing
arrestin-GFP and modified beta2-adrenergic receptor.
For information on this and other applications, email techsupport@compucyte.com
CompuCyte Corporation 12 Emily Street Cambridge MA 02139 USA Phone +1.800.840.1303 www.compucyte.com
Olympus Corporation 3-1 Nishi-Shinjuku 2-chome, Shinjuku-ku, Tokyo Japan 163-0914 www.olympus.com
Transfluor® Assay on the
iCyte™ Imaging Cytometer
Laser Scanning Cytometry
The iCyte™ Automated Imaging Cytometer, developed by CompuCyte Corporation (Cambridge, MA,
USA) is designed to meet the demand in the biotechnology and pharmaceutical markets for
automated high-content cellular analyzers which provide for higher throughput. iCyte has an
architecture suitable for quantitative evaluation of characteristics of compounds measuring biological
responses of cells and tissues. iCyte plays two roles: to acquire brightfield and fluorescent images of
cells and tissues automatically; then, to convert molecular and cellular biological states to numerical
data by analyzing these images within various parameters. These numerical data outputs are further
subjected to processing by statistical analysis software to calculate numerical indexes for quantitative
evaluation of chemical compounds.
Multi-well plates are often used to analyze cultured cells on iCyte. Because iCyte automatically
captures cell images in each well, it is possible to track gradual changes of cellular responses
corresponding to a dilution series of chemical treatment. Laser image scanning is carried out in a nonconfocal manner. Brightfield images are acquired by detecting scattered light with a special detector,
and fluorescent images are obtained by incorporating light emission detected by photomultiplier tubes
(PMT). Although slower than some camera-based systems, this optical architecture is superior in
terms of constituent quantitation.